Ellingham Diagrams
Ellingham Diagrams: Overview
This topic covers concepts, such as, Ellingham Diagram, Applications of Ellingham Diagram & Limitations of Ellingham Diagram etc.
Important Questions on Ellingham Diagrams
In the Ellingham diagram for the solid metals and , gaseous oxides and are:
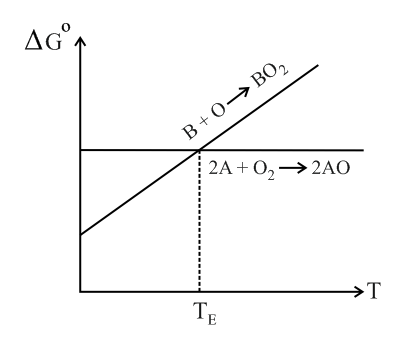
Below point can _____.
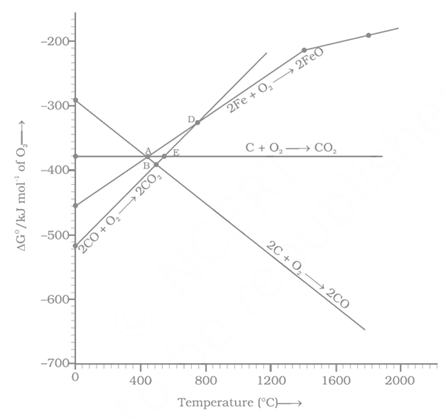
Which of the following metals cannot be obtained by reduction of its metal oxide by aluminium?
Ellingham diagram represents change of
Which one of the following statements is not correct?
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
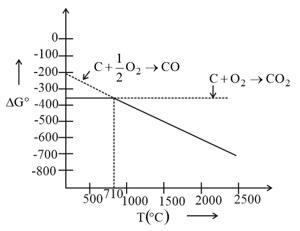
Which of the following are incorrect on basis of above ellingham diagram for carbon?
The correct statement regarding the given Ellingham diagram is:
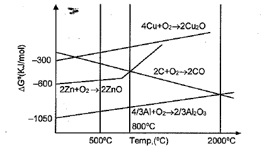
What is the correct statement regarding the given Ellingham diagram?
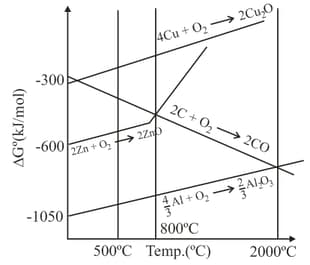
Below the temperature of carbon cannot reduce to because:
For which of the following oxide, carbon reduction is not used for its commercial extraction?
The above reaction is spontaneous at :
The Ellingham diagram below depicts the oxidation of Select the correct statements based on the diagram:
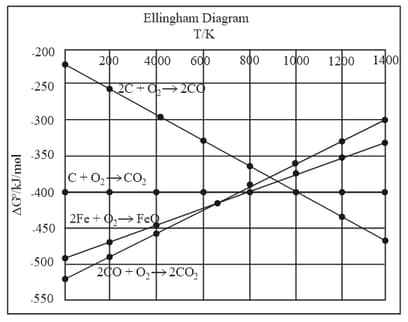
I. can be reduced by below 600 K.
II. can be reduced by below 600 K.
III. can be reduced by above 1000 K.
IV. can be reduced by above 1000 K.
Which of the following factors is not relevant for roasting sulphide ores to oxides and not subjecting the sulphide ores to carbon reduction directly?
Carbon reduction can not be used for extraction of which of the following metals?
Carbon reduction of is not recommended as commercial process of aluminium extraction, because
Select the incorrect statement about Ellingham diagram:
The correct statement regarding the given Ellingham diagram
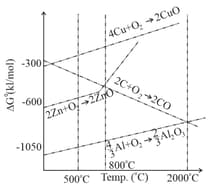
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
versus T plot in the Ellingham's diagram slopes downward for the reaction:
